Blood collection is one of the most fundamental yet critical steps in the diagnostic process. It’s easy to think of it as a simple routine task, but in reality, a single misstep can compromise test results, mislead diagnosis, and even endanger a patient’s life. Pre-analytical errors refer to mistakes that occur before the actual analysis of the sample—during patient preparation, specimen collection, labeling, handling, or transportation. Studies show that nearly 70% of all laboratory errors occur during the pre-analytical phase, making it the most error-prone stage of the testing process.
Understanding the Pre-Analytical Phase
The pre-analytical phase encompasses everything that happens from the moment a test is ordered until the sample reaches the analytical stage in the laboratory. This includes patient identification, preparation, sample collection, labeling, handling, storage, and transportation. Each of these steps plays a vital role in maintaining the integrity of the specimen.
When errors occur in this phase, they can’t be corrected later. A hemolyzed sample, a mislabeled tube, or a sample kept at the wrong temperature can’t simply be “fixed” during analysis—it must be recollected. This not only delays results but also causes discomfort for patients and increases workload for healthcare professionals.
Variability during the pre-analytical stage is one of the biggest challenges in laboratory medicine. Even slight deviations in technique—like shaking a tube too vigorously or not following fasting instructions—can significantly alter test outcomes. That’s why understanding and controlling this phase is the foundation of reliable laboratory testing.
Importance of Correct Patient Identification
One of the most overlooked yet critical steps in blood collection is patient identification. Imagine a scenario where two patients with similar names are admitted in the same ward, and a sample intended for one is mistakenly collected from the other. The results would be attributed to the wrong individual, potentially leading to misdiagnosis, inappropriate treatment, or even life-threatening outcomes. This is not a rare occurrence—studies suggest that patient misidentification errors account for up to 10% of all pre-analytical mistakes in some healthcare settings.
To avoid this, every healthcare professional involved in phlebotomy must strictly follow verification protocols. The “two-identifier rule” is the gold standard: verify the patient’s full name and date of birth (or unique hospital ID number) before proceeding. Never rely solely on room numbers or verbal confirmations, as patients might be confused, sedated, or unable to communicate properly.
Modern healthcare systems often use barcode scanning and electronic medical records (EMR) for specimen identification. These digital systems significantly reduce human error. However, technology is only as reliable as the person operating it. If a barcode is mislabeled or the wrong patient wristband is scanned, the error still carries through the system. Thus, human vigilance remains indispensable.
Regular training, audits, and a culture of accountability are vital. Phlebotomists should be empowered to double-check patient details and encouraged to speak up when discrepancies arise. In the end, accurate patient identification is not just about following rules—it’s about safeguarding lives.

Errors Related to Patient Preparation
Patient preparation might seem simple, but it has a profound impact on the accuracy of test results. Even minor lapses—like drinking coffee before a fasting test or taking medication without consulting a doctor—can drastically alter certain parameters such as glucose, lipid levels, or electrolytes.
For example, if a patient consumes food before a fasting blood glucose test, the results will falsely appear elevated. Similarly, failing to stop certain medications (like corticosteroids or diuretics) can influence test outcomes. This is why clear communication between healthcare providers and patients is critical before collection.
Common preparation errors include:
Not fasting for the required period (usually 8–12 hours)
Consuming alcohol, caffeine, or tobacco before testing
Failing to rest before sample collection (stress can elevate certain hormone levels)
Ignoring the timing of medication administration
To minimize such errors, patients should receive written and verbal instructions well in advance of their test. Clinics can also use pre-test checklists to verify that patients have followed the necessary preparation steps. Phlebotomists must confirm this at the time of collection—never assume compliance.
Remember, an improperly prepared patient leads to misleading results. A little extra care in preparation ensures accuracy, reduces the need for repeat testing, and enhances patient satisfaction.
Improper Choice of Equipment and Tubes
Choosing the correct equipment for blood collection is not merely a matter of convenience—it’s a matter of accuracy. Every type of blood test requires a specific collection tube, which contains additives designed for particular analyses. For instance, a tube with EDTA is used for hematology tests, while a serum separator tube (SST) is used for chemistry panels.
Using the wrong tube or needle can cause sample contamination, clotting, or interference with test results. The color-coded cap system is designed to prevent this confusion. Yet, errors still happen when staff are unaware of or inattentive to these distinctions.
Here’s a quick overview of common tube colors and their uses:
| Tube Cap Color | Additive | Common Use |
|---|---|---|
| Red | None | Serum chemistry, immunology |
| Lavender (Purple) | EDTA | Hematology (CBC, ESR) |
| Green | Heparin | Plasma chemistry |
| Blue | Sodium Citrate | Coagulation studies |
| Gray | Fluoride/Oxalate | Glucose testing |
Additionally, needle size matters. Using a needle that’s too small can cause hemolysis, while one that’s too large might cause discomfort or bruising. The typical range (21–23 gauge) is ideal for most adult collections.
Regular staff training and reference charts near collection areas can help reduce these equipment-related errors. It’s simple: the right tools lead to the right results.
Incorrect Order of Draw
If you’ve ever wondered why phlebotomists follow a specific sequence when filling blood tubes, the answer lies in preventing cross-contamination of additives between tubes. The wrong order of draw can introduce anticoagulants or clot activators into subsequent samples, skewing results.
The standard order of draw, according to CLSI (Clinical and Laboratory Standards Institute), is as follows:
Blood culture bottles (sterile specimens)
Light blue (sodium citrate)
Red or gold (serum tubes with/without clot activator)
Green (heparin)
Lavender (EDTA)
Gray (fluoride/oxalate)
Deviating from this order can, for instance, allow EDTA from a lavender tube to contaminate a serum sample, falsely elevating potassium levels.
To prevent this, phlebotomists should mentally rehearse the order before starting collection and lay out tubes in sequence. Training should include both theoretical understanding and practical exercises to reinforce memory. Some institutions even use color-coded posters in collection areas to guide staff visually.
Remember: the sequence of tubes may seem trivial, but it’s one of the most crucial steps in ensuring test reliability.
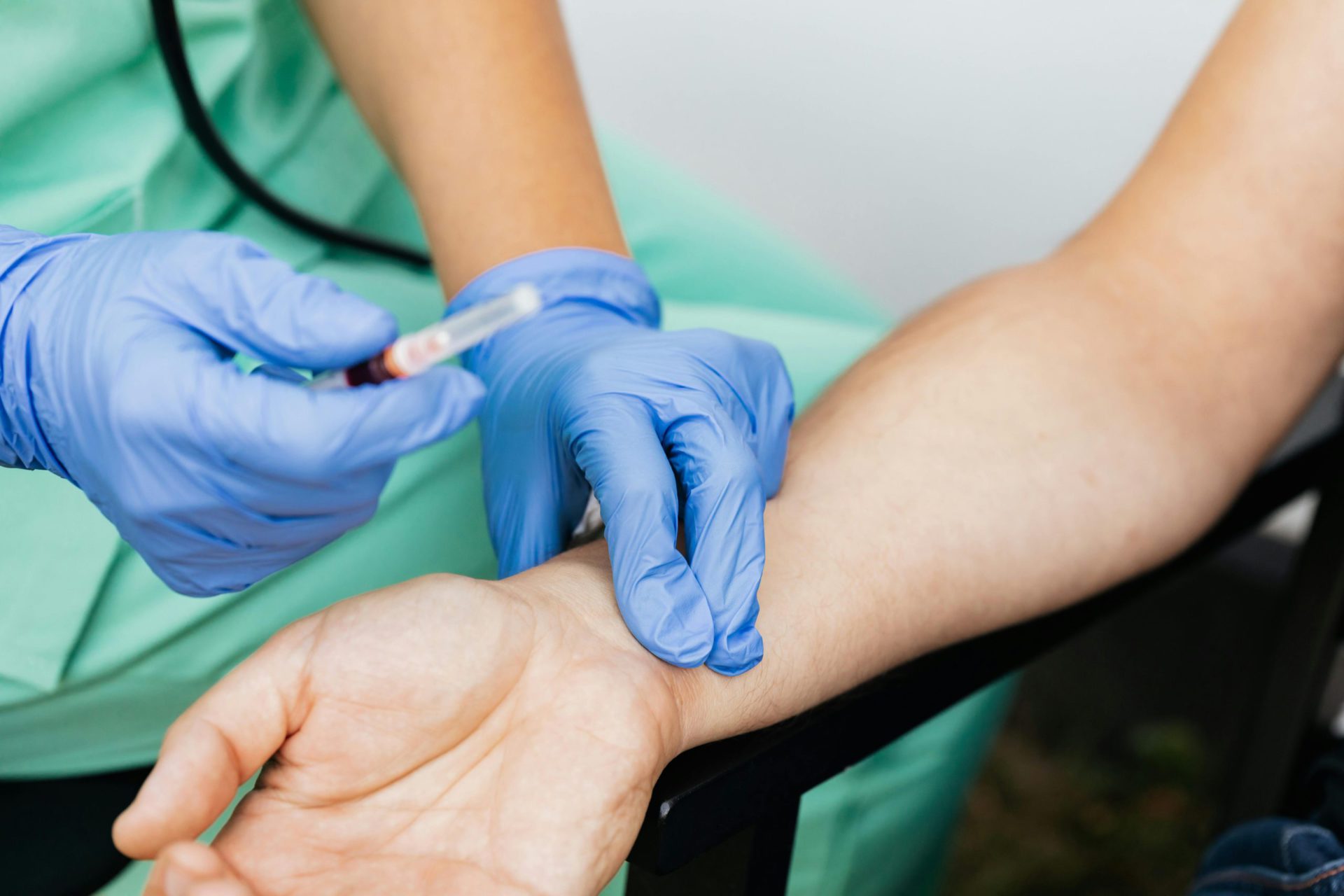
Improper Venipuncture Technique
Venipuncture is a skill that requires both technical precision and patient sensitivity. While it might seem routine for experienced healthcare professionals, even a small deviation in technique can lead to major pre-analytical errors. Common issues such as hemolysis, sample contamination, or insufficient blood draw are often linked directly to poor venipuncture methods.
One of the most frequent mistakes is incorrect site selection. The median cubital vein is the preferred site for most adult patients because it’s large and well-anchored. However, choosing a vein that’s too small, fragile, or close to a valve can cause blood flow issues and result in hemolyzed samples. Additionally, collecting from areas near IV lines can lead to dilution errors since intravenous fluids may mix with the blood sample.
Another technical error occurs during tourniquet application. Leaving the tourniquet on for more than one minute can cause hemoconcentration, leading to falsely elevated values for proteins, calcium, and potassium. Similarly, asking the patient to excessively pump their fist may increase potassium and lactate levels, skewing test results.
Phlebotomists must also pay attention to needle insertion angle and depth. An angle that’s too shallow can cause the needle to slip out, while an overly deep insertion risks puncturing through the vein. Both can cause discomfort, bruising, or sample loss. After blood collection, gently inverting the tube (not shaking) is essential to mix additives without causing hemolysis.
Consistent training and competency assessments help maintain proper technique across staff. Using standardized phlebotomy guidelines, refresher workshops, and peer review can drastically reduce errors related to venipuncture. Ultimately, mastering technique means more than just drawing blood—it’s about ensuring the integrity of every drop collected.
Incorrect Sample Volume
Collecting the right volume of blood may seem like a minor detail, but it’s crucial for accurate results. Underfilled or overfilled tubes can disrupt the additive-to-blood ratio, leading to incorrect readings—particularly in coagulation and hematology tests. For example, too little blood in a citrate tube can result in falsely prolonged clotting times.
Underfilling often happens when phlebotomists withdraw the needle too early, when the vein collapses, or when the vacuum in the tube is weak. Overfilling, on the other hand, can occur if the tube is refilled manually—a practice that should never be done because it compromises sample integrity.
To avoid such errors, always ensure that vacuum tubes are used correctly and filled to their manufacturer’s specified volume. Most tubes have a fill line indicator, and phlebotomists should be trained to check this visually during collection. In pediatric or geriatric patients, where full-volume collection might be challenging, alternative methods or smaller tubes should be used instead of partially filled standard tubes.
Another important consideration is mixing after collection. Undermixing can cause clotting in anticoagulated tubes, while overmixing can lead to hemolysis. Gentle inversion remains the key.
Quality assurance programs should monitor sample rejection rates due to volume issues and use that data to identify recurring problems. A properly filled tube might look like a small thing, but it’s often the line between an accurate report and a misleading one.
Errors in Labeling Samples
Labeling errors are among the most catastrophic yet preventable pre-analytical mistakes. A mislabeled or unlabeled sample can lead to disastrous clinical decisions if test results are attributed to the wrong patient. Unfortunately, these errors still occur due to distractions, multitasking, or rushed environments in healthcare settings.
Proper labeling must always be done immediately after collection, and directly in the presence of the patient. Each label should include the patient’s full name, date of birth or ID number, date and time of collection, and the initials of the collector. Delaying labeling or labeling away from the patient’s bedside opens the door to confusion and mix-ups.
The use of barcoded and electronic labeling systems has significantly reduced human error. However, technology is only foolproof when the right label is applied to the right tube. Scanning patient wristbands and cross-verifying information with the test requisition form should be a mandatory step.
Healthcare institutions can further minimize errors by introducing two-step verification before sending samples to the lab—one by the collector and another by a peer or automated system. Periodic audits and training sessions on labeling compliance can also strengthen accuracy.
Ultimately, correct labeling is not just an administrative step; it’s a safeguard of patient trust. A mislabeled tube can mean the wrong treatment, while a correctly labeled one ensures that each test reflects the right story—the patient’s real condition.
Improper Mixing of Samples
Once blood has been collected, it must be handled with care to preserve its integrity. One of the most overlooked yet impactful steps is the mixing of samples, particularly when using tubes that contain anticoagulants or clot activators. Improper mixing—either too little or too much—can alter the specimen’s composition and invalidate test results.
Undermixing happens when the blood and tube additives (like EDTA, heparin, or citrate) do not blend properly. This can cause clotting in tubes meant for plasma or hematology tests, leading to unusable samples. On the other hand, overmixing or vigorous shaking can destroy red blood cells and cause hemolysis, falsely elevating potassium, lactate dehydrogenase (LDH), and other parameters.
The correct method involves gentle inversion of the tube—usually 5 to 10 times, depending on the type of additive. The motion should be slow and deliberate, ensuring thorough mixing without bubble formation. Tubes should be inverted immediately after collection, as delays increase the risk of microclots forming, especially in anticoagulated specimens.
Another common issue occurs when staff confuse “inversion” with “shaking.” Shaking is too forceful and should never be done. To promote consistency, healthcare facilities should display clear procedural posters near collection areas and conduct periodic skills competency checks to ensure correct mixing practices.
Ultimately, proper mixing isn’t just about following instructions—it’s about protecting sample quality. Think of it like stirring a cup of coffee: too little stirring leaves sugar undissolved, but overdoing it spills the drink. The right balance keeps everything in harmony.
Temperature and Transport Errors
Even after a perfect blood draw, samples can still be ruined if not handled under proper temperature and transport conditions. Some tests require samples to be kept at room temperature, while others demand cooling or protection from light. Ignoring these specifics can lead to degradation of analytes, bacterial overgrowth, or clot formation—rendering the specimen useless.
For instance, blood gases must be transported on ice and analyzed within 30 minutes, while ammonia and lactate levels also require chilling to prevent false elevation. Conversely, tests like potassium and calcium are affected by freezing, which causes cell rupture. Similarly, bilirubin samples must be protected from light, as exposure can degrade the pigment and give falsely low results.
Transport delays are another frequent issue. When samples sit unprocessed for too long, coagulation can occur, and serum separation becomes unreliable. The longer the wait, the more likely analytes begin to break down.
To avoid these errors:
Use insulated transport containers with proper temperature control.
Follow time-sensitive transport protocols for specific tests.
Label samples clearly with “keep chilled” or “protect from light” when applicable.
Maintain a chain-of-custody log to document sample handover times and conditions.
Implementing a specimen transport policy and using temperature monitoring devices are excellent ways to enhance compliance. After all, even the best-drawn blood sample is only as good as the way it’s handled after leaving the patient’s arm.
Centrifugation and Sample Processing Errors
Centrifugation plays a critical role in separating serum or plasma from blood cells. But when done incorrectly—either with the wrong speed, time, or delay—it can lead to inaccurate test results or sample rejection.
If the centrifuge speed is too low, incomplete separation occurs, leaving cellular debris that can interfere with analyte measurements. Too high, and hemolysis can result from the excessive force. The ideal settings vary depending on the type of test and equipment used, but in general, most serum samples require centrifugation at 3000 rpm for 10–15 minutes.
Timing is just as important as speed. Delaying centrifugation allows cellular metabolism to continue, altering levels of glucose, potassium, and enzymes. For example, glucose levels can drop significantly within an hour if the sample isn’t processed promptly.
Phlebotomists and lab staff must also ensure that the centrifuge is balanced properly—placing tubes of equal weight opposite each other—to avoid vibration damage and ensure even separation. After centrifugation, serum or plasma should be separated from cells as soon as possible to maintain sample stability.
Automation systems have made these steps more efficient, but human vigilance is still crucial. Routine maintenance of centrifuges, regular calibration, and adherence to standard operating procedures can minimize processing errors. Remember: centrifugation isn’t just a mechanical step—it’s a precision process that sets the stage for analytical accuracy.
Documentation and Communication Failures
Even when the technical aspects of blood collection are handled perfectly, poor documentation and communication can still derail the entire process. Missing information on requisition forms, illegible handwriting, or miscommunication between phlebotomists and laboratory staff can lead to serious mix-ups and delays.
Documentation errors often include:
Missing patient identifiers
Incorrect test orders or coding
Incomplete collection time records
Unrecorded specimen handling instructions
Each of these can result in misinterpreted or rejected samples. To prevent such issues, all facilities should use standardized requisition forms—ideally electronic—and implement double-check protocols before samples are sent to the lab.
Equally important is the communication between collection staff and laboratory personnel. For instance, if a sample is drawn under special conditions (like fasting, postprandial, or therapeutic drug monitoring), this must be clearly communicated. Otherwise, the laboratory might process the sample under incorrect assumptions.
Institutions can greatly reduce these issues by creating a culture of transparency. Encourage staff to speak up if something seems unclear, and promote teamwork between departments. Communication errors are human, but systems that support cross-checking and information sharing make them much less likely to happen.
Quality Control and Continuous Improvement
The key to minimizing pre-analytical errors lies in continuous quality control and professional development. Regular audits, training sessions, and performance evaluations are essential for identifying weak points and reinforcing best practices.
Quality control should not be limited to the analytical phase; the pre-analytical process must also undergo routine checks. Tracking the number and types of sample rejections provides valuable data for spotting patterns—whether it’s frequent hemolysis, labeling issues, or underfilled tubes.
Implementing Standard Operating Procedures (SOPs) for every step of the collection process ensures consistency, especially in high-volume or multi-staff environments. These SOPs should be reviewed annually and updated to reflect the latest guidelines from organizations like the Clinical and Laboratory Standards Institute (CLSI) and World Health Organization (WHO).
Training programs should include both theoretical knowledge and hands-on workshops, emphasizing real-world scenarios. For example, practicing difficult venipunctures, handling pediatric samples, or managing time-sensitive specimens helps build confidence and competence.
Finally, embracing technology—such as automated labeling, digital data entry, and temperature-tracking systems—can further reduce human error. But technology alone isn’t enough. It’s the combination of human expertise, vigilance, and a commitment to excellence that truly drives quality improvement.
What are the most common pre-analytical errors in blood collection?
The most frequent errors include incorrect patient identification, improper sample labeling, wrong tube selection or order of draw, hemolysis due to poor venipuncture technique, and mishandling during transport or storage. These mistakes can easily be prevented through standardized procedures and proper staff training.
How can I prevent hemolysis during blood collection?
To avoid hemolysis, always use the correct needle size (usually 21–23 gauge), avoid forcing blood through the needle or shaking the tube, and let the alcohol dry before puncture. Blood should flow smoothly into the collection tube using a vacuum system, and samples should be gently inverted—not shaken—after collection.
Why is the order of draw important in phlebotomy?
The order of draw prevents cross-contamination between tube additives. If you draw in the wrong order, anticoagulants or clot activators can transfer to other tubes and alter test results. Following the CLSI-recommended sequence (blood culture → citrate → serum → heparin → EDTA → fluoride) ensures accurate, uncontaminated specimens.
What should be done if a sample is mislabeled or collected from the wrong patient?
If a labeling error or patient mix-up occurs, the sample must never be analyzed. Instead, report the incident immediately, discard the specimen according to policy, and recollect the sample correctly. Documentation of the incident is vital for quality assurance and to prevent recurrence.
How can healthcare facilities reduce pre-analytical errors overall?
Facilities can reduce errors by implementing continuous training programs, using barcoded labeling systems, following strict SOPs, and conducting routine audits. Encouraging a non-punitive reporting culture also helps identify weak points and promotes continuous improvement across teams.
Final Thoughts
Blood collection might seem like a routine task, but it is the first and most crucial step in diagnostic accuracy. Every sample represents a person depending on reliable results for their health decisions. Reducing pre-analytical errors ensures that patients receive the right diagnosis, at the right time, for the right treatment.
With careful attention, clear communication, and commitment to best practices, healthcare professionals can elevate the quality of laboratory services and strengthen patient trust in every result delivered.
Say Goodbye To Waiting Rooms And Long Lines. Speedy Sticks offers at-home testing.